Mixture fraction analysis of combustion products in the upper layer of reduced-scale compartment fires - ScienceDirect

Graph of mole fraction and temperature against mixture fraction from... | Download Scientific Diagram

CHEM 201 - Finding mole fraction from vapor pressure of a mixture with two volatile liquids - YouTube

Question Video: Determining the Mole Fraction of a Gas Given the Mole Fraction of the Other Gas in the Mixture | Nagwa
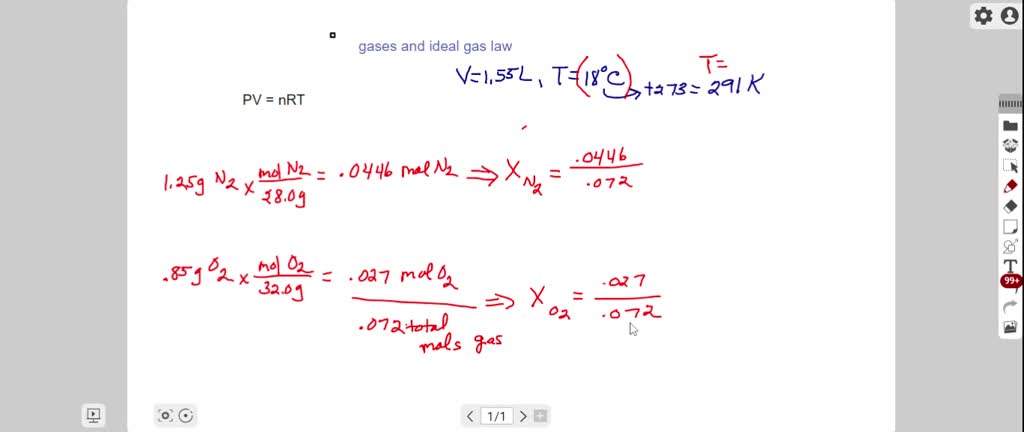
SOLVED: A gas mixture contains 1.25 g N2 and 0.85 g O2 in a 1.55-L container at 18 C. Calculate the mole fraction and partial pressure of each component in the gas mixture.
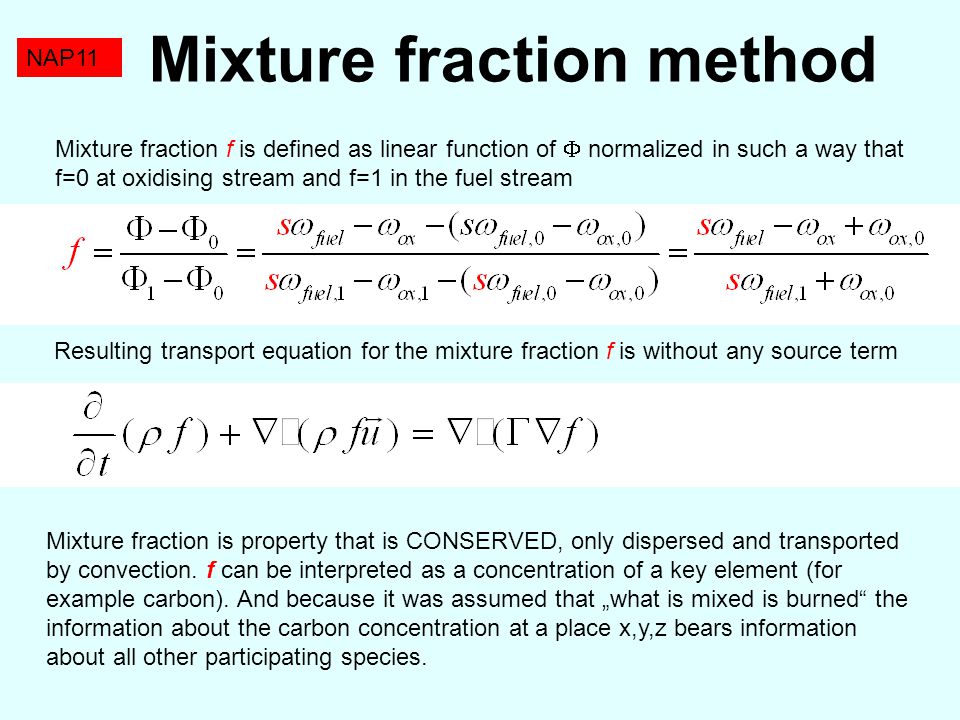
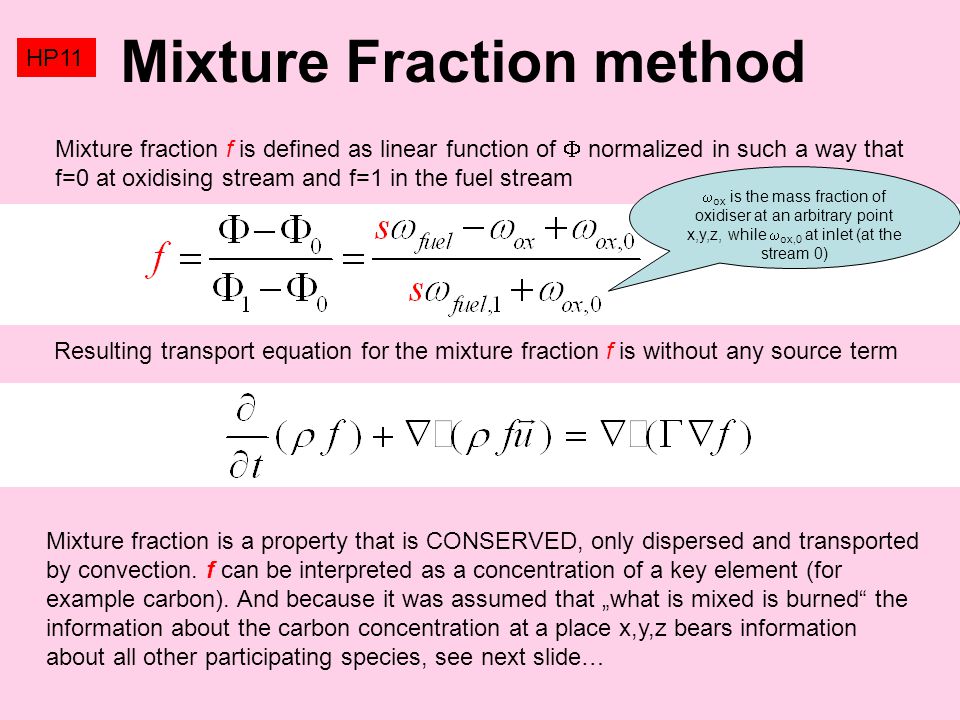
Fundamentals Of Combustion (Part 1) Dr. D.P. Mishra Department of Aerospace Engineering Indian Institute of Technology, Kanpur L

A mixture has 18 g water and 414 g ethanol. The mole fraction of water in mixture is (assume ideal behaviour of the mixture) :

Radial profiles of mean mixture fraction, rms fluctuation of mixture... | Download Scientific Diagram