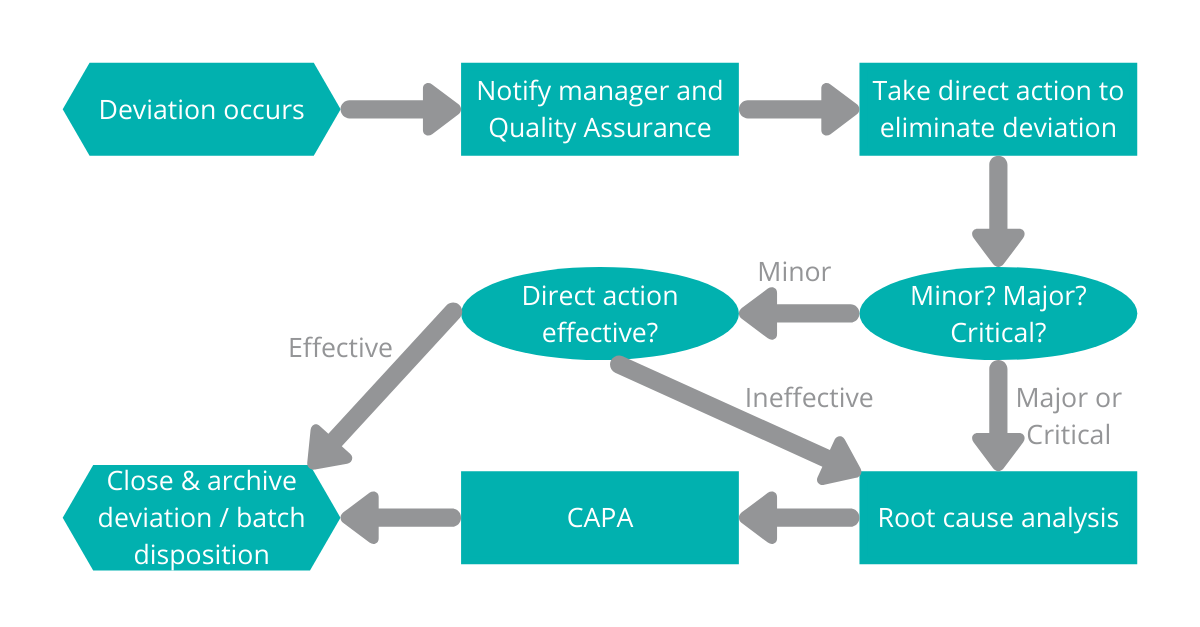
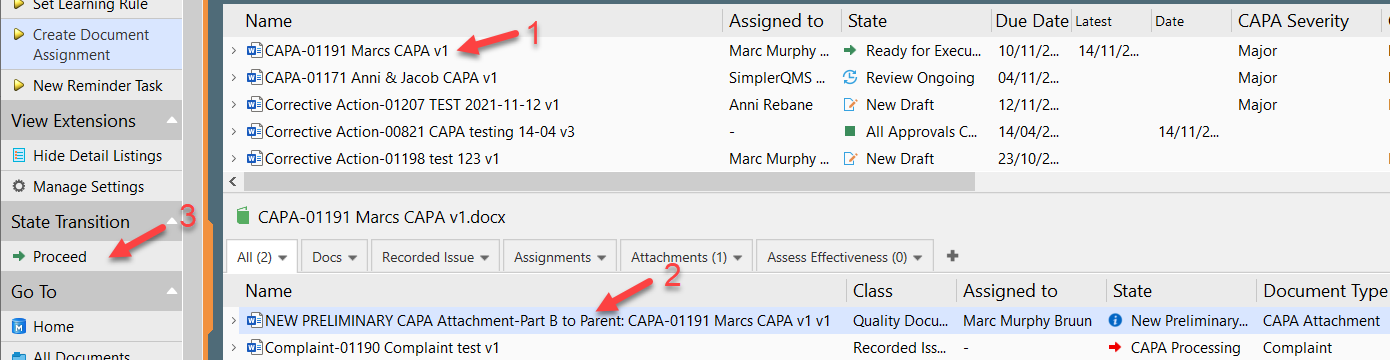
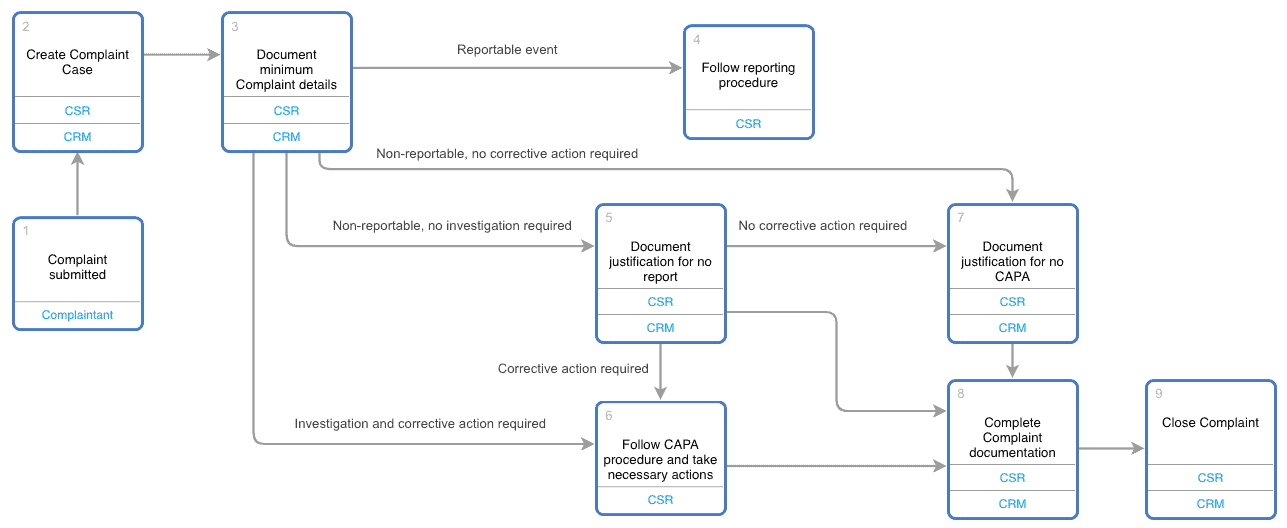
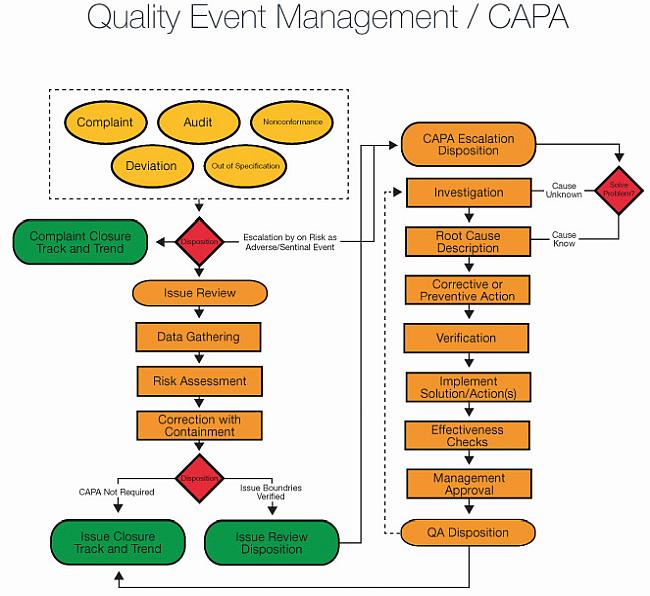
PDF) Investigations and CAPA: Quality system for continual improvement in pharmaceutical industry | Rajesh K . Jain - Academia.edu

Complaint Handling Requirements | CAPA, Change Control, AE Reporting, Recalls : Compliance Training Webinar (Online Seminar) - ComplianceOnline.com

Complaint Handling Requirements - Interrelationship with CAPA, Change Control, Adverse Event Reporting and Recalls, Life Cycle Process Activities - Webinar Compliance